“`html
How to Properly Name Ionic Compounds: A Simple Guide for 2025
Naming Ionic Compounds Basics
Naming ionic compounds is an essential skill in chemistry that allows us to effectively communicate about various substances. Understanding the **naming conventions in chemistry** is crucial, particularly when dealing with different types of ions, including cations and anions. This guide will help clarify the **rules for naming ionic compounds**, giving you a foundational grasp that can lead to successful communication within the scientific community. We will dive into various strategies and examples to equip you with the knowledge to name both binary and ternary ionic compounds accurately.
Cation Naming Rules
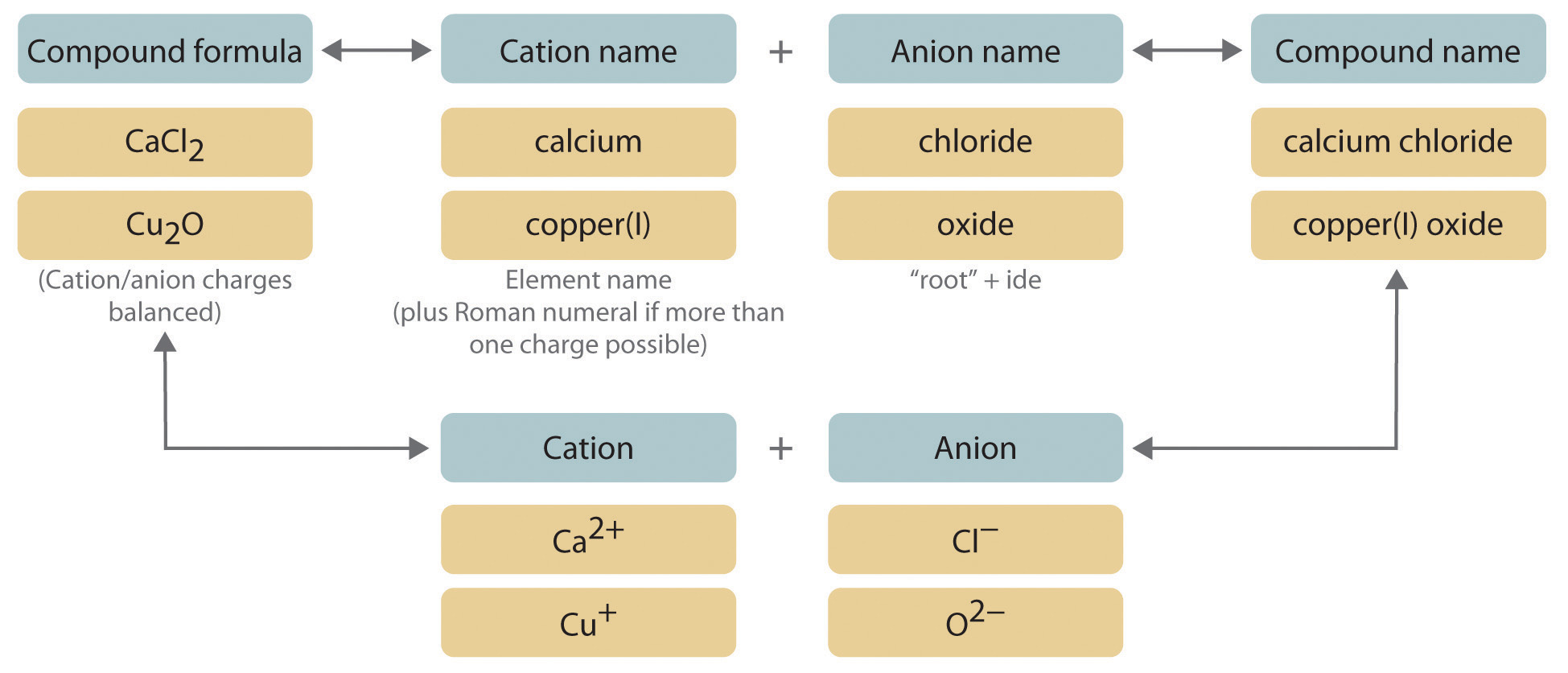
Cations are positively charged ions, often derived from metals. The **cation naming rules** depend on whether the metal can form multiple oxidation states. Simplistically, cations from Group 1 and 2 of the periodic table are simply named by their element name, such as sodium (Na+) or calcium (Ca2+). However, for **transition metal ions** like iron (Fe), which can have more than one oxidation state, it’s important to use **naming with Roman numerals** to indicate the charge, such as iron(II) for Fe2+ or iron(III) for Fe3+. This systematic approach aids in the understanding of ionic compounds, correlating names with their respective charges and structures.
Anion Naming Rules
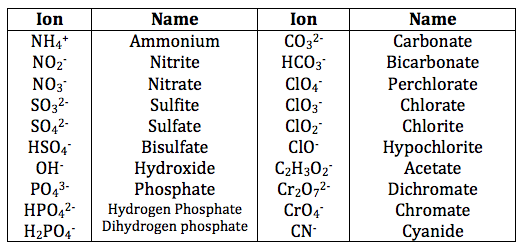
Anions, or negatively charged ions, are usually derived from nonmetals. The **anion naming rules** often involve changing the suffix of the element’s name: for example, chlorine becomes chloride (Cl–), and oxygen becomes oxide (O2-). Furthermore, polyatomic ions like sulfate (SO42-) and nitrate (NO3–) require special attention due to their distinct naming conventions. Understanding these rules is foundational for learning how to name ionic compounds correctly, providing clarity when encountering complex combinations.
Identifying Cations and Anions
When facing an unknown ionic compound, the first step is identifying the **cations and anions** it contains. Using the periodic table can aid in recognizing common ions and their charges. Once the ions are identified, applying the naming conventions for cations and anions discussed above will help in forming the correct name for the compound. For instance, if you have a compound composed of sodium ions (Na+) and chloride ions (Cl–), the compound would be sodium chloride. Familiarization with a **common ionic compounds list** can further speed up this identification process, as it highlights frequently encountered compounds.
Understanding Ionic Compound Nomenclature
To master **ionic compound nomenclature**, it’s essential to first understand the fundamental characteristics of standard ionic compounds. These compounds typically consist of a metal and a nonmetal held together by ionic bonds formed through the electric attraction between the positive and negative ions. Familiarity with the **ionic bond formation** allows for a deeper comprehension of their properties, which directly impacts the naming process. This section will outline the systematic methods for naming simple and complex ionic compounds to ensure clarity when addressing more advanced naming challenges.
Naming Binary Ionic Compounds
Binary ionic compounds consist of exactly two different elements—one cation and one anion. The naming convention follows a straightforward approach: name the cation first and then the anion, changing the anion’s ending to “-ide.” For example, the compound formed by combining potassium (K+) and oxygen (O2-) becomes potassium oxide (K2O). This formula reveals that two potassium ions are required to balance the charge from one oxide ion, resulting in a neutral compound. Understanding how to accurately write the formulas is critical; hence this directory of **naming binary ionic compounds** will reinforce the fundamental rules.
Naming Ternary Ionic Compounds
Ternary ionic compounds involve three or more elements and often include at least one **polyatomic ion**. When naming these compounds, the polyatomic ion retains its name. For instance, calcium sulfate (CaSO4) is composed of the calcium cation and the sulfate anion. It is pivotal to become proficient in recognizing the different polyatomic ions since they often differ significantly from simple cations and anions. Furthermore, it’s recommended to reference a **common cation names and common anion names list** throughout this learning process to enhance fluency in ionic compound nomenclature.
Naming Double Ionic Compounds
Double ionic compounds contain two different cations or anions working in opposition. An example would be aluminum hydroxide, which contains both aluminum (Al) and hydroxide (OH–) ions. The naming of these compounds is similar to that of ternary compounds but requires further emphasis on the use of parentheses to clarify which ions are present when multiple units of polyatomic ions are involved, as seen in aluminum sulfate [Al2(SO4)3]. Mastering this aspect of **naming double ionic compounds** not only aids comprehension of complex formulae but also enhances your overall understanding of ionic compounds.
Advanced Nomenclature Techniques and Practice
As you advance your knowledge of ionic compounds, it becomes imperative to explore the **naming strategies for ionic compounds** that can simplify the learning and application process. Hands-on practice is critical for reinforcing the systematic naming and writing formulas for ionic compounds. This section will provide resources for exercises that enhance your skills by employing **naming ionic compounds practice problems** and worksheets tailored to challenge your understanding.
Writing Formulas for Ionic Compounds
Learning to write formulas for ionic compounds is just as important as learning how to name them. Employing a straightforward approach in determining the ionic charges established from the composition enables you to craft the correct formula. For instance, recognizing that calcium forms a +2 cation (Ca2+) and chloride forms -1 anions (Cl–) allows you to determine that you need two chloride ions to balance the charge, leading to the formula CaCl2. Regularly practicing these coordination techniques is vital in **formula writing for ionic compounds** that will aid in comprehension and retention of concepts discussed previously.
Common Mistakes in Ionic Naming
Errors are an inherent part of learning, especially in the context of naming ionic compounds. A frequent mistake is the misuse of prefixes for compounds since they apply primarily to covalent compounds, not ionic ones. This results in confusion, as ionic and covalent compounds follow distinct rules. Additionally, memorizing certain exceptions, like polyatomic nonmetals and transition metals with Roman numeral indication, can be challenging without diligent practice. Utilizing resources such as **naming ionic compounds worksheets** can provide substantial practice opportunities to overcome these pitfalls.
Online Tools and Resources
Counting in today’s digital age, many online tools and platforms provide interactive resources for **naming ionic compounds**. These tools incorporate quizzes and educational modules designed to engage and enhance students’ chemistry learning experiences. From virtual labs experimenting with ionic structures to interactive naming quizzes, leveraging educational technology can make learning complex ionic nomenclature accessible and enjoyable. Evaluating these resources can contribute significantly to your understanding and capability to name compounds effectively.
Key Takeaways
- Understand the **binary and ternary ionic compounds** conventions for naming.
- Employ **naming with Roman numerals** for certain **transition metal ions**.
- Practice identifying **cations and anions** accurately to facilitate correct naming.
- Utilize diverse **online tools for naming compounds** to reinforce learning.
- Recognize common errors to prevent misapplication of naming rules.
FAQ
1. What are the basic rules for naming ionic compounds?
The basic rules for naming ionic compounds include identifying the cation followed by the anion, where the anion’s name often ends in “-ide.” For transition metals, it’s crucial to indicate the oxidation state with Roman numerals, and when complex ions are involved, maintaining their original names is essential.
2. How do you differentiate between binary and ternary ionic compounds?
Binary ionic compounds consist of two elements—one cation and one anion. On the other hand, ternary ionic compounds include three or more elements, typically involving at least one polyatomic ion. This distinction is critical for applying the relevant naming conventions properly.
3. What are some common polyatomic ions you should know?
Some common polyatomic ions include sulfate (SO42-), nitrate (NO3–), and hydroxide (OH–). Being familiar with these ions is essential when naming ternary compounds, as their names often remain unchanged in various combinations.
4. Why do we use Roman numerals in naming ionic compounds?
Roman numerals are used to indicate the charge of transition metals that can exist in multiple oxidation states. For instance, iron can have a +2 or +3 charge. To specify which iron compound you are referring to, you would write iron(II) for Fe2+ and iron(III) for Fe3+.
5. How can I improve my skills in naming ionic compounds?
Improving your skills in naming ionic compounds can be achieved through consistent practice using worksheets and online quizzes. Engaging with interactive learning tools, visualizing ionic structures, and discussing naming strategies within study groups can also help solidify your understanding of nomenclature in chemistry.
“`
